Difference between revisions of "Anorexia anx/anx mutant mice"
(→Re-Amplification: added detail) |
(added range of concentrations so far) |
||
| Line 43: | Line 43: | ||
Measure DNA quantity/quality with Nanodrop. | Measure DNA quantity/quality with Nanodrop. | ||
| − | * yield was | + | * yield was 60 - 1100 ng/ul genomic DNA. |
===PCR=== | ===PCR=== | ||
| Line 51: | Line 51: | ||
For 50 ul reaction: | For 50 ul reaction: | ||
| − | * | + | * 25 ul Gotaq |
| − | * | + | * 10 ul mixture of 5 uM forward/reverse Tyro3 primers |
| − | * | + | * 14 ul dH<sub>2</sub>O |
* 1 ul of genomic DNA | * 1 ul of genomic DNA | ||
| Line 59: | Line 59: | ||
PCR: 95C for 1min, then 30 cycles of: 95C for 1min, 72C for 2min. (note annealing and extension steps combined into 2 min at 72C). | PCR: 95C for 1min, then 30 cycles of: 95C for 1min, 72C for 2min. (note annealing and extension steps combined into 2 min at 72C). | ||
| − | + | ====Gel==== | |
| − | |||
| − | ===Gel=== | ||
2% agarose in TAE (2 g agarose in 100 ml TAE, microwave 2 min at 50% power; add 10 ul of SYBR safe 10000x while cooling). Make mini-gel with 12-tooth comb | 2% agarose in TAE (2 g agarose in 100 ml TAE, microwave 2 min at 50% power; add 10 ul of SYBR safe 10000x while cooling). Make mini-gel with 12-tooth comb | ||
| Line 67: | Line 65: | ||
load 10 ul of 50bp ladder; 10 ul of PCR product + 2 ul purple loading dye | load 10 ul of 50bp ladder; 10 ul of PCR product + 2 ul purple loading dye | ||
| − | run at 90V for 30 min (or 65V for 60 min for sharper bands). Red dye front migrates at about | + | run at 90V for 30 min (or 65V for 60 min for sharper bands). Red dye front migrates at about 370bp, similar to bromophenol blue. |
| − | ===PCR clean-up=== | + | ====PCR clean-up==== |
[https://omegabiotek.com/product/pcr-clean-up-kit-e-z-n-a-cycle-pure/ E.Z.N.A. Cycle Pure Kit (V-spin)] yields 30 ul in dH<sub>2</sub>O [ because worried about pH of dH<sub>2</sub>O, now trying kit elution buffer which I think is Tris-Cl pH 8.5] | [https://omegabiotek.com/product/pcr-clean-up-kit-e-z-n-a-cycle-pure/ E.Z.N.A. Cycle Pure Kit (V-spin)] yields 30 ul in dH<sub>2</sub>O [ because worried about pH of dH<sub>2</sub>O, now trying kit elution buffer which I think is Tris-Cl pH 8.5] | ||
| Line 75: | Line 73: | ||
Measure DNA quantity/quality with Nanodrop. | Measure DNA quantity/quality with Nanodrop. | ||
| − | * yield was | + | * yield was 20 - 65 ng/ul clean PCR product |
===Re-Amplification=== | ===Re-Amplification=== | ||
| Line 94: | Line 92: | ||
Incubate at 37 C for 1 hour, heat inactivate 65 C for 20 min. | Incubate at 37 C for 1 hour, heat inactivate 65 C for 20 min. | ||
| − | NEBiolabs says to mix digest with purple dye with 0.5% SDS to separate protein from DNA. SDS may interfere with SYBR Safe, so may need to post-stain the gel. | + | NEBiolabs says to mix digest with purple dye with 0.5% SDS to separate protein from DNA. SDS may interfere with SYBR Safe if stain mixed in the gel, so may need to post-stain the gel. |
Revision as of 14:56, 29 November 2024
Anorexia anx/anx mice Jackson Lab strain 000624.
Mutation mapped to a 0.2 cM interval residing between the markers D2Mit133 and Jojo5 chromosome 2 (Chr 2: bp 118, 889, 896–120, 175, 1081) (Lindfors et al., 2011).
Possible genes affected
Lindfors 2011 PMID 22025706: NADH dehydrogenase (ubiquinone) 1a-subcomplex (Ndufaf1)
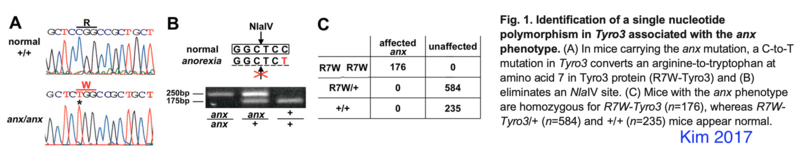
Kim 2017 PMID 28093506: tyrosine kinase receptor Tyro3 which they conclude is not the anx-mutation but a strain specific modifier of anx-phenotypes
Abnormalities Table
Tyro3 Genotyping
TYRO3 protein tyrosine kinase 3 (Tyro3) mutation (C19T-Tyro3 mutation) in anx/anx eliminates a NlaIV restriction site.
- primers forward 5′-GATGGCGCTGAGGCGGAGCATG and reverse 5′-CGCGGCCGGAGGTCTGGCAG ; annealing temperature 72C
- predicted PCR product size: 280bp equal to Mus musculus strain C57BL/6J chromosome 2, GRCm39, where forward primer is 119630270-119630291 and reverse primer is 119630549-119630530
- subsequent digestion with NlaIV
- wildtype -> 175 bp fragment, anx/anx -> 250 bp product (eyeballing from Kim 2017)
Genotyping 1998 mice
- 2024-1-17 Tyro3 primers ordered from FSU BIO Core
- 2024-1-18 NlaIV ordered from NEBiolabs, along with 50bp ladder and purple dye.
- 2024-6-6 Promega GoTaq colorless master mix ordered from Fisher (suspect green master mix interferes with digest)
DNA extraction from tails/spleens
E.Z.N.A. Tissue DNA Kit yields 200 ul in elution buffer.
Measure DNA quantity/quality with Nanodrop.
- yield was 60 - 1100 ng/ul genomic DNA.
PCR
PCR master-mix: Promega Gotaq colorless master mix.
For 50 ul reaction:
- 25 ul Gotaq
- 10 ul mixture of 5 uM forward/reverse Tyro3 primers
- 14 ul dH2O
- 1 ul of genomic DNA
PCR: 95C for 1min, then 30 cycles of: 95C for 1min, 72C for 2min. (note annealing and extension steps combined into 2 min at 72C).
Gel
2% agarose in TAE (2 g agarose in 100 ml TAE, microwave 2 min at 50% power; add 10 ul of SYBR safe 10000x while cooling). Make mini-gel with 12-tooth comb
load 10 ul of 50bp ladder; 10 ul of PCR product + 2 ul purple loading dye
run at 90V for 30 min (or 65V for 60 min for sharper bands). Red dye front migrates at about 370bp, similar to bromophenol blue.
PCR clean-up
E.Z.N.A. Cycle Pure Kit (V-spin) yields 30 ul in dH2O [ because worried about pH of dH2O, now trying kit elution buffer which I think is Tris-Cl pH 8.5]
Measure DNA quantity/quality with Nanodrop.
- yield was 20 - 65 ng/ul clean PCR product
Re-Amplification
To get higher concentration of DNA for digest, repeat PCR using 1 ul of clean PCR product and clean up.
- yield was xxx - xxx ng/ul clean re-amplified PCR product
(could also pool repeated original PCR Rxns from genomic DNA, but that seems inefficient. Re-amplifying risks PCR artifacts/mutations, though ...)
Digestion
- 1 ul NlaIV (2 units)
- 5 ul rCutSMart Buffer 10x
- 1 ug clean Tyro 3 PCR product
- H2O to 50 ul
Incubate at 37 C for 1 hour, heat inactivate 65 C for 20 min.
NEBiolabs says to mix digest with purple dye with 0.5% SDS to separate protein from DNA. SDS may interfere with SYBR Safe if stain mixed in the gel, so may need to post-stain the gel.