Difference between revisions of "LacZ"
| Line 1: | Line 1: | ||
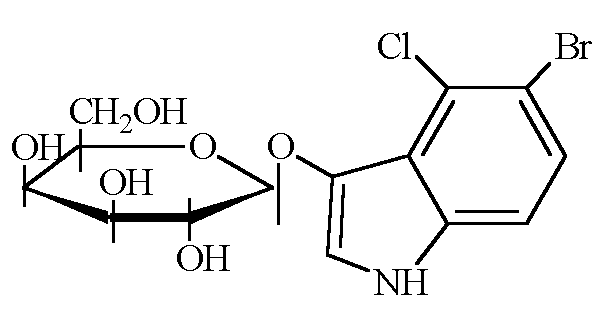
5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside: | 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside: | ||
| + | |||
| + | [[Image:xgal.gif]] | ||
Revision as of 14:41, 22 August 2007
5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside:
http://www.bio.davidson.edu/courses/genomics/method/xgal.gif
Cleavage by beta-galactosidase yields a dihalogen-substituted indoxyl, 5-bromo-4-chloro-3-indoxyl:
Oxidation in the presence of an electron acceptor yields a relatively insoluble indigoid dye:
Oxidants used in histochemistry
Potassium ferri- and ferrocyanide. ferricyanide is the electron acceptor; ferrocyanide is "provided in equimolar amount ... to give a system with a poised oxidation potential." (Holt and Withers, 1958, p. 524).
Nitroblue tetrazolium Used as a test for tuberculosis? PMID 4151974 The NBT test assesses the ability of immune cells to convert NBT to a blue dye. In chronic granulomatus disease, the immune cells are missing the ability to generate reactive oxygen via phagocyte NADPH oxidase, so those white blood cells fail the NBT test.
Performic acid PMID: 1099403
Cepko, C., Ryder, E., Fekete, D.M., and Bruhn, S. Detection of B-galactosidase and alkaline phosphatease activities in tissue. Online: http://genepath.med.harvard.edu/~cepko/protocol/xgalplap-stain.htm. An excellent brief discussion of X-Gal histochemistry. Much of the material presented here was derived using their references.
Studies in Enzyme Cytochemistry
I. Principles of cytochemical staining methods. Holt and Sullivan, Proc. R. Soc. London B 148 (1958) 465-480. A nice theortical discussion of reaction rates and diffusion rates through tissue as it relates to localization of a colormetric stain. Very useful as a tutorial on how to calculate diffusion, but not much help on LacZ staining per se. A key conclusion is that in order to get a good "localization factor" ( F ), the reaction rate generating the insoluble dye product must be much faster than the diffusion rate of the soluble substrate precursor. (therefore, oxidation rates of indoxyls must be increased with catalysts like ferricyanide).
II. Synthesis of indigogenic substrates for esterases. Holt and Sandler, Proc. R. Soc. London B 148 (1958) 481-494. Describes the synthesis and reactions of a series of indoxyls, including 5-bromo-4-chloro-indoxyl acetate (one of the indoxyl acetates(VII), Table 8 p. 493) . Note that acetate groups are attached to the indoxyls so that esterase enzymes could be used to rapidly form the dyes. Demonstrates that the NH group helps attach to local proteins to enhance intracellular localization.
III. Relationships between solubility, molecular association, and structure in Indigoid dyes. Holt and Sandler, Proc. R. Soc. London B 148 (1958) 495-505. the effects of different substitutions, configuation of the dimers, hydrogen bonding, molecular packing and crystallization on silutbility in water and different lipid environments. The presence of halogen substitutions cause less crystalline products (compared to indigo itself), which is good for a staining dye.
IV. Kinetics of aerial oxidation of indoxyl and some of its halogen derivatives. Cotson and Holt, Proc. R. Soc. London B 148 (1958) 506-519. Instead of an electron acceptor to aid in oxidation, oxygen itself can be used (aerial oxydation). Aerial oxidation requires a basic environment (i.e. pH 8.0 or above; p. 512). At pH 11.0, 87% of 5-bromo-4-chloro-indozyl should be converted to dye (one of the higher conversion rates reported; p. 514). Aerial oxidation also produces H202 as a by product, which might come in handy if an additional stain can be used to visualize the hydrogen peroxide (p. 515) . H202 causes a lot of artifacts, however, if there are peroxidases present, because the peroxidases will rapidly oxidase the indoxyls. There are two problems with aerial oxidation: 1. The lacZ enzyme prefers an acidic environment (pH 6.0), not a basic environment. 2. Aerial oxidation while fast is not fast enough to deposit the insoluble dye before it can diffuse (p. 518)
V. An appraisal of indigogenic reactions for esterase localization. Holt and Withers. Proc. R. Soc. London B 148 (1958) 520-532. This paper uses the best substrate identified in the preceding papers, 5-bromo-4-chloro-indoxyl acetate , to localize esterases in various rat tissues. The reactions were carried out at pH8.5 in Tris in 0.2 M NaCl using 5 mM potassum ferri- and ferrocyanides as oxidants (p. 522). At pH 6.0 and below, staining was diffuse even with high oxidant concntrations; basic conditions were best (pH 8.5 and above (p. 525-6). [Also tried aerial oxidation, with too much red blood cell staining and non specific staining; p. 524]. Notes that the NH group is important for protein binding, and the 5:4' substitutions enhance the binding to protein greatly (with disubstitutions, the dye leaves the cytoplasm and diffuses into fat.)
Note p. 523: "Exposure of stained sections to formalin vapour or solution must be very brief since formaldehyde reacts with many indigoid dyes to give soluble products and loss of stain."
Recommends ferrocyanide, and notes that it inhibits red blood cell staining "presumably because of the chemical similarity to cyanide"